Cyanide and Heavy Metal Removal
A comparison of different chemistries with emphasis on an innovative new treatment method.
© 1994, Steven A. Holtzman, Advanced Chemical Technology, Inc.
Cyanide and heavy metals removal from wastewater streams are only a few of the extremely important fields required to insure the protection of global bio diversity through responsible environmental management. With the enactment of the "Clean Water Act", and the need for a more sustainable level of industrial development, ever more stringent levels of water quality in industrial wastewater are required.
Waste Segregation
Prior to treatment, it is necessary to segregate the different wastewater streams according to the type of treatment that will be required. As a minimum, it is imperative to segregate all cyanide bearing wastes, and all chrome bearing wastes, from any other waste streams. If possible, it is also desirable to segregate acidic streams, from basic streams, and highly chelated streams.
Cyanide Removal
The first step in any waste treatment process is to remove cyanide. Cyanide is used to complex metals such as cadmium, gold, platinum, etc. so that they will remain in solution at high pH ranges.
There are three different methods for removing cyanide. The first, used in Europe but not in the US, is acid hydrolysis. In this process, the pH of the solution is lowered with sulfuric acid and the resulting hydrogen cyanide is captured and recovered. This process is extremely dangerous due to the production of cyanide gas, and is not used in this country.
The second method is to oxidize the cyanide to cyanate with ozone. This method has been demonstrated to work in the laboratory but has not been as successful in the field. The reason for this is most likely due to the short half life of ozone.
The most common method of cyanide destruction is known as alkaline chlorination. This is usually accomplished in two stages. In the first stage, the solution pH is raised to approximately 10 - 11. Bleach is added to achieve an ORP level of approximately +500 mv. The solution is then allowed to react for 30 - 60 minutes while the cyanide is oxidized to cyanate. In the second stage, solution pH is lowered to 8.5 - 9.0. Additional bleach is added until the ORP is at a level of approximately +800 mv. This reaction takes 45 - 90 minutes and will oxidize the cyanate to CO2 and nitrogen. At this point the cyanide bearing waste streams can be mixed with the normal non-cyanide bearing waste streams for additional treatment.
It is important to note that cyanide can form complexes that are not amenable to alkaline chlorination. It is therefor important to prevent these complex cyanides from forming in the first place. The metals that usually cause these complex cyanides to form are, iron, chrome, and nickel. Once these complexes have formed it may be possible to break them by adding excess bleach and increasing the reaction time.
Chrome Destruction
Chrome exists in two valence states; +6 and +3. The +6 or hexavalent state is very soluble over a broad pH range. This is also the most toxic form of chrome. The +3, or trivalent state is insoluble at higher pH levels and is fairly easy to remove from water.
The first step in removing chrome is to reduce the hexavalent chrome to the trivalent form. This is usually accomplished by reducing the pH to 2.5 - 3.0 with sulfuric acid and then adding sodium metabisulfite to achieve an ORP of +300 mv. This reaction is fairly quick and will normally be accomplished in approximately 15 - 20 minutes.
An alternative method of reducing chrome is done at a high pH with the addition of hyrosulfide. Eliminating the use of sulfuric acid is an advantage; however this method is relatively expensive.
After all of the hexavalent chrome has been reduced to trivalent chrome, the solution can be added to the normal metal bearing waste streams for additional treatment.
Mercury Removal
Many types of mercury treatment technology have been reported and the majority are based upon laboratory or piolet scale studies. Hydroxide precipitation is ineffective, with soluble levels exceeding 75 mg/l mercury over a pH range of 3.5 to 11.5.
Sulfide precipitation is the most common method of removing mercury. This method has all of the problems associated with sulfide precipitation that are discussed later in this report.
Precipitation with sodium borohydride has also been reported. This method has many problems associated with it that include the formation of explosive hydrogen gas and the tendency for the mercury to go back into solution. Again, this method is discussed later in this report.
The most successful method is to use organometallic precipitation. This method can be used to reduce the level of mercury to 10 µg/l or less. Frequently we are able to achieve results of non-detectable levels. This process is discussed in more detail later in this report.
Chelated Wastes
The most common chelating agents found in metal bearing wastes are cyanide, EDTA, NTA, ammonia, and citric acid. Chelates function by complexing with metal ions thereby keeping them in solution at elevated pH levels. In order to remove the metals it is necessary to break the chelated complexes. In the past, this has typically been accomplished by either adding large quantities of coagulants such as alum or ferric chloride or by raising or lowering the pH to extreme levels. The use of large amounts of coagulants is called "salting out", and although it will work, it creates a large volume of "RECRA" sludge that is typically hauled to landfills. Later in this paper we will discuss an extremely effective and new process for removing chelated metals that significantly reduces the creation of sludge.
Traditional metal removal processes
Hydroxide Precipitation
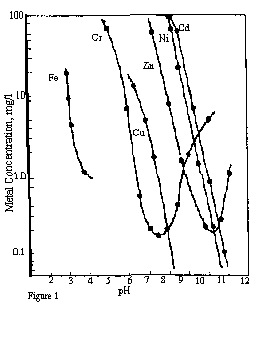 Traditionally, dissolved metals have been removed from water by the process of hydroxide precipitation. Since most metal hydroxides are insoluble, it would appear easy to remove metals by this process. The main problems associated with this process are that hydroxides of different metals have different pH levels for minimum solubility (see Figure 1 at right), and the reactions are of an equilibrium type, i.e., some of the metal hydroxide will disassociate with the resulting metal ions going back into solution.
Traditionally, dissolved metals have been removed from water by the process of hydroxide precipitation. Since most metal hydroxides are insoluble, it would appear easy to remove metals by this process. The main problems associated with this process are that hydroxides of different metals have different pH levels for minimum solubility (see Figure 1 at right), and the reactions are of an equilibrium type, i.e., some of the metal hydroxide will disassociate with the resulting metal ions going back into solution.
For example, nickel has its lowest solubility at a pH of 10.5 - 11.0, but chrome is at a minimum solubility at a pH of 7.5 - 8.0. Therefore, this method of metal removal can leave high levels of some metals still in solution or require an additional neutralization step. Additionally, mercury cannot be removed by this method at all.
The most common reagents used for hydroxide precipitation are caustic soda, lime, and magnesium hydroxide
Sulfide precipitation
Soluble metals can also be removed by precipitating them as a sulfide by the addition of sodium sulfide to the solution. This method yields more complete metal removal than hydroxide precipitation but can easily leave toxic sulfides in solution. This method is much more expensive than hydroxide precipitation since the excess sulfides are usually regulated and the resulting sludge may be difficult to landfill. Therefore, it is not as widely used as hydroxide precipitation.
Modern metal removal processes
Carbamates
Carbamates are chemical reducing agents that can be obtained as either sodium dimethyldithiocarbamate or sodium diethyldithiocarbamate. Carbamate precipitation is again an equilibrium reaction that does not go to completion. Metal residuals of 1.0 - 1.5 mg/l can usually be obtained. Carbamates are not effective at acidic pH levels and are not always effective at treating chelated wastes.
Sodium Borohydride
Sodium borohydride is an extremely strong reducing agent and can reduce both chelated and non chelated metals. This process has the advantage of producing the least amount of sludge of any process but it has a number of disadvantages that almost always preclude its use in an efficient and cost effective system.
A major disadvantage, is that unless the liquid is removed from the sludge immediately, metals tend to go back into solution with the water. Another problem is that pH control is critical. Explosive hydrogen gas is evolved at acidic pH values.
High cost of this reagent has also been a problem, and as a result, it has been very difficult to justify its use.
Organometallic Precipitation
The synthesis of organometallic and coordination compounds has attracted interest among chemists during the past few decades. Organometallic compounds are organic molecules containing at least one atom of a metal bonded to a carbon atom. A familiar example of this class of substance is tetraethyl lead, which was often added to the gasoline that fuels internal-combustion engines. Other organometallic compounds include catalysts used in plastic manufacture and in organic synthesis.
In 1991 Steve Holtzman, pioneered a new more environmentally responsible method of removing heavy metals. This process revolves around the formation of insoluble organometallic compounds formed by reacting metal bearing wastes with a proprietary organic agent.
By forming specific types of insoluble organometallic compounds, all regulated metals can be reduced to non-detectable levels. The process is easily controlled with an inexpensive ORP controller and can adapt to changing levels of contaminants in the waste stream influent.
This process works over an extremely broad pH spectrum (1.5 - 12) and has the ability to break most chelates in extremely high concentrations. Since the metals are precipitated as an organometal complex at all pH values, there is no problem with different levels of solubility based on pH. The only reasons for pH control are to make certain that the waste effluent is in a range that is allowed by the discharge permit and to allow the polymer flocculents to be in a pH range where they are effective.
The volume of sludge produced is comparable to that produced by borohydride. The sludge volume produced by this method and caustic soda is approximately 50% of the sludge that is produced by using caustic soda alone and precipitating the metals as hydroxides. If magnesium hydroxide is used as the caustic agent, sludge volumes can be as low as 25% of the sludge volume produced by hydroxide precipitation. figure 2 illustrates this.
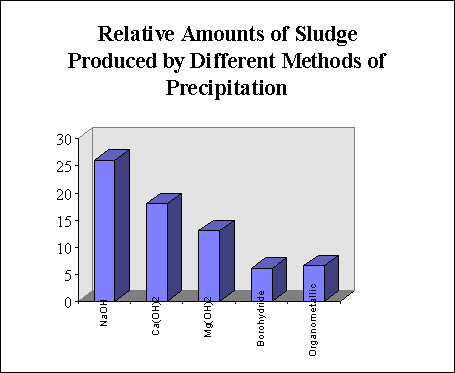
Figure 2
The best treatment approach that we have found is to first do any cyanide or chrome destruction that may be required. Adjust pH (if found necessary during bench testing) and add the organic reducing agent on a 2.5 to 1 stoichiometric basis. The pH is next adjusted to 7.5 - 8 with magnesium hydroxide and any coagulants required are added. Anionic polymer is then added to flocculate the metals. Metal levels in the effluent can be "dialed in" according to customer requirements. Typical residuals of all regulated metals are 0.01 mg/l or less.
Figure 3 shows a typical waste treatment facility using alkaline chlorination for cyanide destruction, sodium metabisulfite for chrome destruction and, organometallic precipitation for the removal of heavy metals.
Typical results comparing hydroxide precipitation to organometallic precipitation are illustrated in Figure 4.

Figure 3
(click to enlarge)
Comparison of metal residuals between hydroxide precipitation and organometallic precipitation
| Metal |
Effluent Limit |
Hydroxide Precipitation |
Organometallic Precipitation |
| Cadmium |
0.07 |
0.06 |
<0.002 |
| Chromium |
1.71 |
1.04 |
<0.01 |
| Copper |
2.07 |
0.75 |
<0.01 |
| Lead |
0.43 |
0.34 |
<0.01 |
| Nickel |
2.38 |
1.97 |
<0.01 |
| Silver |
0.24 |
0.01 |
<0.01 |
| Zinc |
1.48 |
1.20 |
<0.01 |
Figure 4
Bibliography
Brady, James E. and Humiston, Gerard E. General Chemistry: Principles and Structure. Wiley, 4th ed., 1986.
Caglioti, Luciano and Giacconi, Mirella. The Two Faces of Chemistry. MIT, 1983
Hill, John W. Chemistry for Changing Times. Burgess, 4th ed., 1984.
McGraw-Hill Dictionary of Chemical Terms. McGraw, 1985.
Goodman, Murray and Morehouse, Frank. Organic Molecules in Action. Gordon and Breach, 1973.
Solomon, T. W. Organic Chemistry. Wiley, 5th ed., 1992.
Sykes, Peter. A Guidebook to Mechanism in Organic Chemistry. Longman, n.d. Halsted, 6th ed., 1986.
Stevens, Malcolm P. Polymer Chemistry: An Introduction. Oxford, 1990.
"Chemistry, Organic," Microsoft ® Encarta. Copyright © 1993 Microsoft Corporation. Copyright © 1993 Funk & Wagnall’s Corporation

